Success factors for more resilience in the pharmaceutical and chemical industry.

Authors:
Taner Memet Ali - Head of Solution Architecture, GUS ERP GmbH.
Olaf Buck - Head of New Customer Projects, GUS ERP GmbH.
So far, the chemical and pharmaceutical industry has come through the COVID-19 pandemic relatively unscathed. While 40% of German chemical companies still reported a severe shortage of orders in spring 2020, according to the German Chemical Industry Association (VCI), this figure had fallen to just 18% in the first quarter of 2021. This is because demand has often simply shifted towards other or new products. The more resilient a company is, the more flexibly it can deal with such rapid changes in direction. Resilience, in turn, comes from largely digitalized processes and a high level of data integrity.
Agile processes as a success factor
Disinfectants were suddenly in short supply in spring 2020 at the start of the pandemic. For some chemical producers, this opened up a new market not despite the crisis, but precisely because of it. Prerequisite: they had to have the necessary raw materials or make them available. In addition, many fundamental questions had to be clarified as quickly as possible: Can the products in demand even be realized on the basis of the existing infrastructure and processes? If so, how quickly and at what cost? What (new) partnerships are needed? How will the supply chains change as a result? Could profits be made with the new products and to what extent?
Abrupt change in sales and products
Basically, the more digitalized a company is and the more integrated its digital systems and processes are, the faster and more agile companies can deal with such sudden changes of course. But what does this look like in concrete terms for an individual chemical and pharmaceutical manufacturer?
The respective ERP system plays a decisive role here. A modern, process-oriented and flexible ERP system manages all relevant data for planning sales, purchasing raw materials or recipes. With a central workflow engine, it also links the company's internal processes, for example between sales, production and logistics. In this way, it makes it possible to operate with parts lists or recipes. It also synchronizes the data from product development with procurement and delivery sources or with warehouse stocks and available raw materials. Artificial intelligence can also be used to reliably forecast sales in the same process step.
Online sales on the rise
However, it is not only the product range in demand that has been restructured due to the pandemic. In addition, sales and supply chains are clearly moving towards online platforms. As local stores often had to close, sales shifted towards online stores and sales platforms such as Amazon Market Place, where chemical companies can market their products directly. On the other hand, B2B trading exchanges, such as the Swiss start-up kemiex, experienced a significant boost, where (preliminary) products are traded, new partner relationships are established and new supply chains are created. But how can connectivity to these e-commerce and B2B platforms be achieved at the touch of a button or with just a few configurations?
The real challenge here is not the mere technical connection to such platforms. Rather, the provider company's ERP system must have all the essential information and be able to display it in a linked format. This includes, for example, parts lists, recipes, replenishment times in the form of lead times in purchasing, production, warehousing, quality control, internal transportation, or the delivery times of potentially available suppliers or new logistics channels for B2C business. Some of this data comes from internal systems, but some also comes from external sources.
Accordingly, the respective ERP system must have so-called "connectors" that integrate external information sources via suitable interfaces. In addition to the technical connection via open standards such as REST, uniform semantics are required to exchange the data.
Overcoming media discontinuities
The ERP solution GUS OS-Suite, for example, implements such connections via the GUS-OS Digital Hub Service. This is a cloud-based service that makes the processes of the ERP solution accessible outside the company via REST interfaces in order to integrate suppliers, mobile employees and even machines and IT systems into the company's own ERP environment. No master or transaction data is lost and no sensitive data is stored in the cloud. This is because only those parts and functions of the ERP system that are necessary for communication are opened up to the outside world. Furthermore, all communication is encrypted.
However, the reality in companies without a modern ERP system is often quite different: There are still numerous media disruptions, particularly between the individual departments in the company - for example between production, laboratory and quality assurance. In some cases, the data flow is still paper-based. This is also shown, for example, by the constantly increasing number of warnings from the FDA (Food & Drug Administration), whose inspectors are increasingly focusing on inadequate data flows with many media breaks. Process-oriented ERP systems provide a remedy here by closing media disruptions and reliably digitizing cross-departmental processes.
Increasing focus on quality management
In this context, companies have also recently invested more heavily in quality management - on the one hand in special systems tailored to this purpose, but also in connecting them to their respective ERP systems, where all key data converge. As a rule, pharmaceutical and chemical producers are affected by numerous national and international regulations. As a result, the responsible employees must be able to react quickly if something changes in the specified QM processes.
Above all, they must be able to recognize deviations from the specifications and then assign them to CAPA measures (CAPA, Corrective Actions, Preventive Actions). Such anomalies include, for example, OOS (out of specification) results, batch-related deviations or complaints. This assignment requires a structured and centralized database, which can be provided first and foremost by the respective ERP system.
The same applies to cost control: modern ERP systems now have fully integrated financial accounting, audited in accordance with GoB/GoBs/GDPdU and certified in accordance with IDW880, including asset accounting and liquidity planning. The aim is the immediate availability of all costs - from contribution margin and batch costing to flexible cost accounting. Ultimately, company resilience also means being able to quantify exactly what planned production changes will ultimately cost on the one hand and what added value they will bring on the other.
Dashboards - process-oriented
A key requirement for agile ERP systems can be derived from such an overall view: Central control stations, "dashboards" or "cockpits", which collect and cumulate all data and visualize it for the respective roles and responsibilities in the company, are suitable for controlling and monitoring processes by users. From this condensed display, users can then drill down to the detailed data and information they need. This not only enables an optimal workflow connection, but also a user-centered and needs-oriented data presentation.
In addition to the availability and visualization of data and processes in dashboards, an ERP control station should not only be able to display the data, roles and responsibilities. It is also important to link them together, in short: the process view. Because only those who take a process-oriented approach instead of a purely document-oriented one are able to quickly and flexibly correlate and change all key production and sales processes - across departments.
This is only possible if those responsible in the company no longer think in terms of individual functions or departments, but rather in terms of interrelated company processes. Last but not least, it is essential that the respective ERP system maps the industry-specific features of the process and chemical industry, for example industry-specific authorization matrices or compliance rules. All of this also improves the integrity and usability of the data.
Integer data, transparent processes
The importance of data integrity and process-oriented thinking and action for resilient and reliable production processes is currently being demonstrated by vaccine manufacturer BioNTech. The Mainz-based company was and is ahead of most established players in the market when it comes to vaccine development and production. The most important reason for this lead is that the company consistently focuses on the smooth exchange of data and a high level of process transparency. Together with its partner Pfizer, BioNTech has thus far been able to precisely calculate and meet delivery deadlines within a short period of time. This example shows how companies can remain capable of acting with the help of process orientation and high data quality, even if markets, products, sales channels or supply chains should change abruptly once again.
Resilience in the chemical and pharmaceutical industry - the top 5 success factors
1) Process orientation: workflow control with multi-stage approval processes and their documentation
2) End-to-end transparency: from the individual machine to the profit and loss account in order to be able to plan and simulate changes holistically
3) Integrated QM: Linking of all quality-relevant processes such as supplier evaluations, laboratory tests, procurement and production processes
4) Cost control: The ERP system or an external solution in conjunction with it offers fully integrated financial accounting, audited in accordance with GoB/GoBs/GDPdU and certified in accordance with IDW880, which makes all costs available at the touch of a button and enables flexible cost accounting.
5) Future-proof technologies: through continuous further development of the solutions, for example with regard to IoT, AI or the secure and flexible connection of business partners, sales platforms, etc.
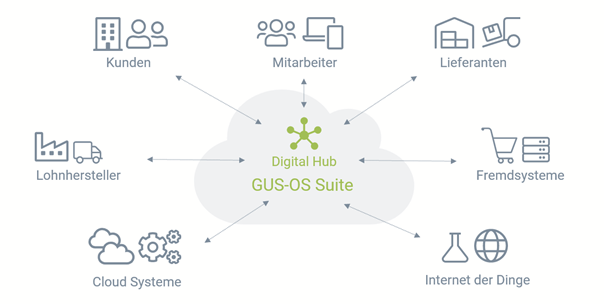
Illustration: The Digital Hub of the GUS-OS Suite connects the ERP system with external platforms
About the GUS Group
The GUS Group(www.gus-group.com) develops and implements fully integrated software solutions for the process industry (pharmaceuticals, medical technology, chemicals, cosmetics, food, food retail) and logistics. The product portfolio supports the entire business cycle - from enterprise resource planning (ERP) to supply chain management (SCM), producer accounting (EVS), sales management and geomarketing, customer relationship management (CRM), laboratory information management (LIMS), quality management, finance/controlling and business intelligence through to document management. The GUS Group's approximately 400 employees support more than 1,300 mostly medium-sized companies as well as corporations and public authorities.


