QM manual - GUS ERP GmbH
You have opened the home page of our Quality Management Manual. Our manual consists of a public and a non-public area. In the public area you can move around as you wish; we have marked links to the non-public area with "not public". This is confidential, further information about our company and its processes, access to which is restricted to our employees.
I hope you enjoy reading it.
(Version 2.2 from 22.11.2023)
Scope
The scope of our quality management system extends to all processes and organizational units of the
- GUS ERP GmbH
Josef-Lammerting-Allee 20-22
50933 Köln - GUS ERP GmbH - Hamburg Branch
Heidenkampsweg 73
20097 Hamburg - GUS ERP GmbH - Munich branch
Claudius-Keller-Str. 3c
81669 Munich - GUS Schweiz AG
Sonnenstrasse 5
9000 St. Gallen
Switzerland
Conformity
Our quality management system meets the requirements of the DIN EN ISO 9001:2015 standard
DQS certificatedownload
Not applicability
Since we do not use any test equipment that needs to be calibrated, the requirements of point 7.1.5 of the DIN EN ISO 9001:2015 standard (resources for monitoring and measurement) are not relevant for our company and are not applicable.
(Version 1.4 from 16.01.2018)
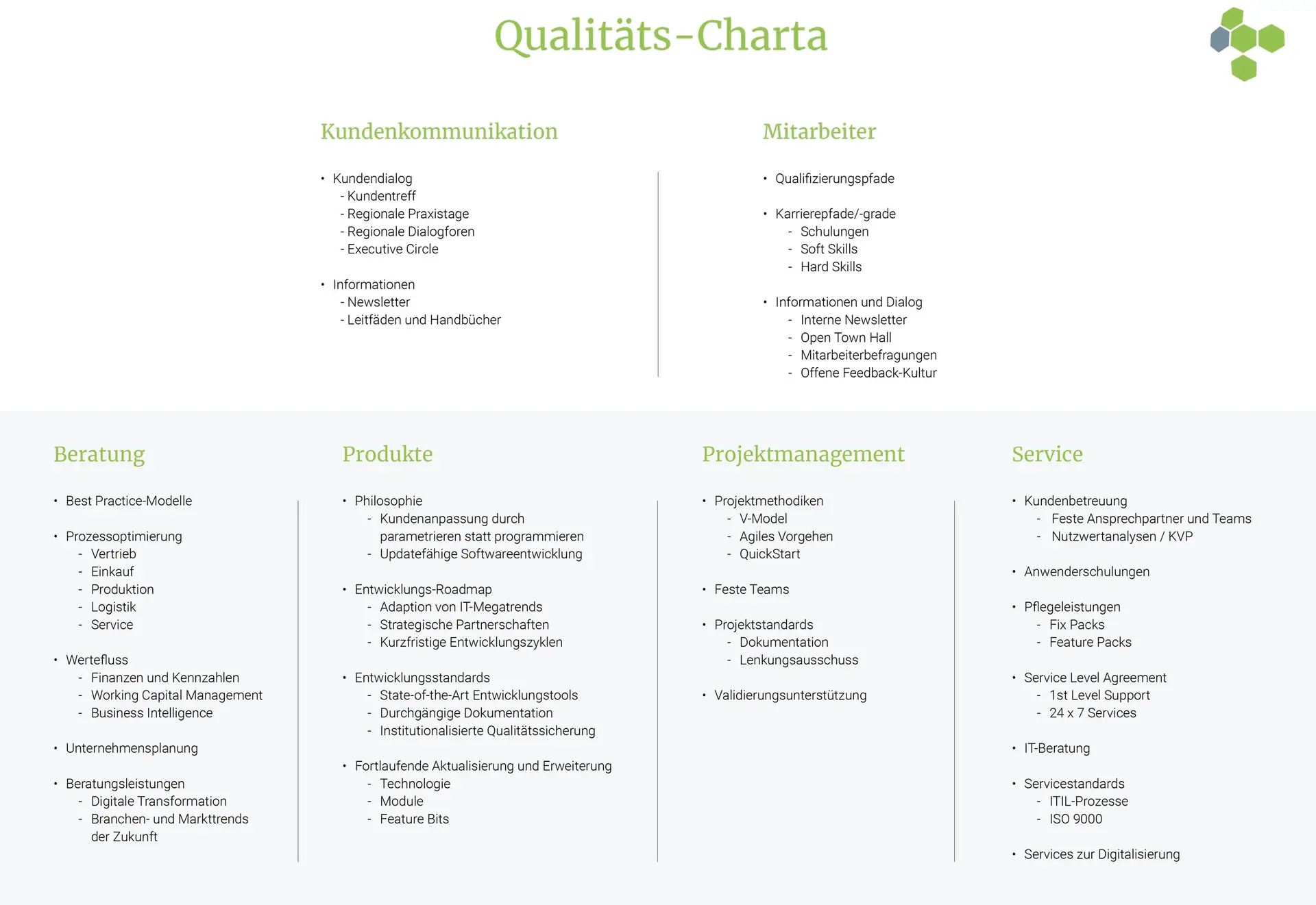
Probably no other industrial sector sets such high quality requirements as the companies in the pharmaceutical, food and beverage, chemical, cosmetics, biotechnology and related logistics industries. The products of these companies touch the immediate sphere of people's lives and thus their health, well-being and quality of life.
Therefore, the requirements for development, procurement, manufacturing, quality control and distribution as well as the accompanying information go far beyond the standards of an industry standard such as ISO 9001:2015. In addition to the guidelines of the "Good Manufacturing Practice" Good Manufacturing Practice (GMP) as well as related regulations such as Good Laboratory Practice (GLP), Good Storage Practice (GSP) in the pharmaceutical sector, there are countless legal or official requirements: Whether it is the provisions of the German Medicines Act (AMG), the German Ordinance on the Manufacture of Medicinal Products and Active Pharmaceutical Ingredients (AMWHV), Chapter 21 of the Codes of Federal Regulations (CFR) or - for the food sector - the Food Hygiene Act and EU Regulation 178/2002.
In addition, trade guidelines such as International Food Standard (IFS) and British Retail Consortium (BRC) Global Standard-Food must be followed. These frameworks all focus on the care of the company and its employees.
An important quality assurance instrument recognized by the industry is also validation. Here, the GUS Group complies with the regulations of the Pharmaceutical Process Engineering Working Group (APV), which are set out in the APV guideline "Computer-aided systems".
Our diligence also includes the selection of production equipment and software systems. We consider it a compliment to our own quality orientation that numerous companies in the life sciences industries have chosen our solutions to support their quality-oriented business processes. In doing so, we have made our customers' quality specifications the benchmark for the quality goals of our own business processes. This diligence is formulated in our quality charter and is thus an obligation for each and every employee - and this applies to all areas of responsibility:
- in the development and testing of software systems
- in the implementation of customer-specific specifications
- in support and system assistance in sales
- in the selection of system components
- in employee qualification
- in the company organization
Commitment, personal responsibility and professional competence of the individual are the pillars of our success. The requirements and wishes of our customers determine our objectives. The organization of our company with its structures, goals and regulations creates the right environment for an efficient community. Accordingly, each individual is responsible for the results of the work assigned to him or her within the framework of his or her competencies and responsibilities.
Quality objectives
We have defined and established process indicators (non-public) for steering, analyzing and improving our quality targets.
These are collected and analyzed on a regular basis. The process indicators are determined at different intervals depending on the indicator. The key figures form the basis for the monthly management meeting. There, the figures are analyzed and, if necessary, control measures are taken.
(Version 1.3 from 16.01.2018)
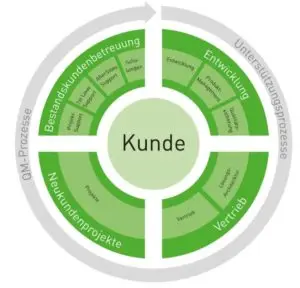
All our core processes in the areas of
- Development
- Distribution
- New customer projects
- Existing customer care
focus on our customers. In the following graphic you will get information about our processes:
(Version 1.4 from 13.02.2012)
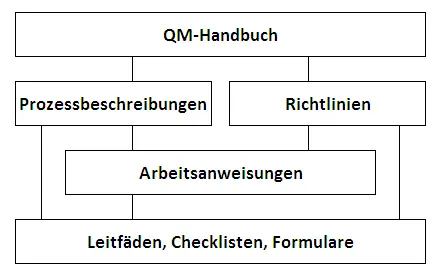
Structure of the QM default documentation
In addition to this management manual, the standard documentation of our management system consists of process descriptions, guidelines, work instructions and checklists (not public), which describe process sequences and contain specifications for their proper implementation. Furthermore, these documents refer to applicable forms, checklists and guidelines (not public). All QM standard documents are clearly identifiable with their revision status.
Updating, reviewing and approving QM prespecification documentation.
The respective process owners are responsible for updating the QM default documentation. The following applies to the signature regulation for QM default documents:
⇒ Creation ⇒ Author
⇒ Check ⇒ Process owner, process participation, QM representative
⇒ Release ⇒ Spokesman of the managing directors
The QM organizational unit is responsible for managing the QM standard documents (not public). This department ensures that the valid version of the standard documentation is available and known to all employees of the company. As a matter of principle, each QM default document always retains its validity until a new version is officially released or the document is withdrawn. The intended optimization and/or modification of a process is also subject to the specifications of released QM default documents, i.e. the application of existing rules. In coordination with the QM representative, however, a process owner can define a pilot project and/or an exception that is not subject to the existing regulations. Without exception, however, this is subject to the written approval of the QM representative. If, in an individual case, it is not possible to apply existing rules (e.g., because a situation is not taken into account in the specification) without this being coordinated with the QM representative (e.g., because of a necessary decision at short notice), this must be documented in writing by the person responsible for the process, stating the initial situation and the considerations that have led or will lead to the deviation, and communicated to the QM representative.
Issuance of QM default documents
The QM manual is public and may be passed on as a copy to customers and other interested parties. However, these are exclusively information copies, which are not subject to the ongoing modification and exchange service. They correspond to the current status only when they are issued. Other documents, such as process descriptions and work instructions (not public), contain know-how of our company and are therefore to be treated confidentially. Their release to parties outside our company requires the approval of the company management in each individual case.
Data and records
The type and scope of necessary records are specified in our QM default documentation. Inspection results are recorded for the respective processes and products. Such records serve as proof of the inspection carried out as well as documentation of the results. These records are regularly evaluated by the persons responsible for the processes in order to obtain indications of changes and weak points in the organization.
(Version 1.3 from 16.01.2018)
Planning and control
At the beginning of the financial year, the key performance indicators of recent years that are critical to success are analyzed. Based on this, a plan is drawn up for the coming financial year together with the expected market development. This is broken down to the individual months together with the plan parameters. These plan figures and plan parameters form the basis for the monthly TARGET/ACTUAL analysis that takes place at the managing directors' meeting. Within the scope of this analysis, all economic and performance-influencing parameters are processed and subjected to a comprehensive analysis. This enables GUS to react quickly to changes in the market and to take the necessary measures for corporate management.
Process key figures
We have defined and established (non-public) process indicators for the management, analysis and improvement of our quality targets. These are collected and analyzed on a regular basis. The process indicators are determined at different intervals depending on the indicator. The key figures form the basis for the monthly management meeting. There, the figures are analyzed and, if necessary, control measures are taken. In regular management reviews, commercial and quality-relevant data of the company are analyzed and discussed, which allow conclusions to be drawn about the performance of the company's processes. Inputs for the management review are:
- Results of internal audits,
- Results of supplier audits,
- Feedback from customers (reports to the hotline),
- Process metrics,
- Economic key figures
- Target/actual comparisons with plan data
- Status of preventive and corrective actions,
- Changes that affect the management system,
- Recommendations for improvements.
- Feedback from relevant interested parties
- Results of monitoring and measurements
- Services from external providers
In the event of anomalies, appropriate measures are initiated to respond as promptly as possible to the weaknesses identified in the company. The results and determinations of the assessment are recorded and communicated to those responsible. The implementation of agreed measures is monitored by the quality officer and the management circle.
Internal audits
In internal audits (not public), we regularly review the implementation of our specifications in practice and the knowledge of our employees about the regulations affecting their work. Particular attention is paid to the development of process optimizations; our employees can provide valuable information on this from their practical work. Audits are systematically planned and carried out by trained, competent personnel. The results of internal audits are documented. Deviations from specifications and identified weaknesses are backed up with appropriate measures to eliminate the causes. The effectiveness of such corrective and preventive measures is reviewed in follow-up audits.
(Version 2.3 from 22.11.2023)
The verification of our management system is carried out by several independent control procedures and bodies.
Supplier audits
In supplier audits, customers and interested parties review our quality management system. More and more companies, especially from the pharmaceutical industry, are following the recommendations of the GAMP guidelines, which expressly recommend that pharmaceutical companies conduct supplier audits. Since we are constantly working on optimizing our processes, information from external audits is particularly valuable, as it is not subject to our natural "operational blindness". We have received numerous suggestions for process and quality optimization from supplier audits and have been able to implement them successfully.
Results from supplier audits (excerpts)
19.04.2023 BGS Beta Gamma Service GmbH & Co. KG
Conclusion: The supplier GUS ERP GmbH remains an approved supplier of BGS.
11.11.2021 G. Pohl-Boskamp GmbH & Co. KG
The audit revealed that GUS ERP GmbH operates a well-functioning quality management system and has extensive experience with the requirements for software implementation in a GMP-regulated environment. The processes are comprehensively documented.
21.11.2019 medac GmbH / oncomed manufacturing a.s.
The release of the GUS Group as a qualified supplier and service provider is recommended.
09/03/2019 Medios Manufaktur GmbH
- GUS ERP GmbH maintains a very well functioning quality management system certified according to ISO 9001:2015.
- The audit results confirm the very high professional competence of the employees in the context of software development and validation in the GxP-regulated environment.
- Already in the project phase for the introduction of the GUS-OS ERP software, GUS ERP GmbH was able to convince with its many years of experience in software implementation in the pharmaceutical industry.
19.07.2019 Pharmpur GmbH
GUS services Pharmpur GmbH in the preparation of an ERP system for Pharmpur ́s business processes. The company is very well suited for this purpose. GUS demonstrated to have a very good quality management system in place. The system is based on ISO9001:2015. Pharmaceutical processes are known to GUS.
22.11.2016 Wagener & CO. GmbH
During the audit, the discussions held and the subsequent review of the enclosed documents, there were no reasons whatsoever not to use GUS as a supplier for GMP-relevant software.
27.09.2016 Schaper & Brümmer
Due to the positive audit results, the release of GUS ERP GmbH as a qualified supplier for the development, implementation and maintenance of the ERP system GUS-OS Suite is recommended by the auditors [...]. [...]
GUS ERP GmbH operates a well-functioning QM system in accordance with ISO 9001:2008, which was audited on a random basis. [...]
The audit results prove the necessary experience and GMP competence of the company for the development, implementation and maintenance of the OS-ERP software. [...]
The company was also able to demonstrate that its extensive experience in the pharmaceutical industry underpins its competence.
02.02.2016 aniMedica
Summary / Assessment
During the audit, GUS presented itself as a very structured company. Based on many years of experience in the GMP area through previous projects and the available inspection results, GUS fully complies with the requirements of Annex 11 "Computerized system" of the EU GMP guidelines and the requirements of the Aide-Memoire "Monitoring of computerized systems" of the ZLG and can therefore be classified as a qualified supplier of ERP software.
24.06.2015 Medac
The company presented itself with a strong focus on the quality management system in the audit carried out for the requalification of the CIS. [...]. The overall impression was very positive. GUS has expanded and further optimized its quality processes.
All audit participants provided the requested information immediately and explained it in detail. The requested documentation was available in full.
GXP compliance is still in place. The FDA compliance highlighted in the last audit was spotlighted and could be confirmed at these points. [...]
04.03.2015 Excella
The GUS Group AG & Co KG has extensive project and audit experience in theGxP regulated market.
The audit formally confirmed that the requirements for software development in the context of implementation projects are fulfilled in accordance with good practice and the requirements of ISPE GAMP5.
In addition, the project personnel have a good knowledge of GMP. The QM system is well documented and has proven to be capable of mapping the software development process and, in particular, ensuring traceability. However, the formal correctness of the working methods does not necessarily allow conclusions to be drawn about the overall success of the project.
03.12.2014 Protina
The audit was conducted in a positive atmosphere.
The audit was very well prepared. The introduction to the audit was well structured.
The results demonstrate the necessary experience and GMP competence of the company for the development, implementation and maintenance of the OS-ERP software.
The company GUS Group was able to demonstrate in the audit that customers from the GMP-regulated industry have carried out successful audits in recent years. The company was able to demonstrate that extensive experience in the pharmaceutical industry underpins its expertise. It could be shown that the first projects with pharmaceutical companies with medical devices have been successfully realized.
The employees have many years of experience in the GxP market, a high level of understanding of the validation requirements and identification with the company.
Compared to the ERP market (DACH), GUS is very well positioned to meet the requirements of the GxP-regulated industry.
08.06.2014 BAG
The supplier audit carried out at GUS ERP GmbH, Cologne site, has shown that the audited areas
- Quality management system
- Software development process
- Test procedure
- Support and maintenance
- FDA 21 CFR Part 11 Compliance
complies with the principles and requirements set out in the ISPE GAMP5 guidelines.
The supplier audit also confirms that GUS ERP GmbH has the necessary experience in the GxP environment and that the development, implementation and maintenance of the GUS OS Suite software is carried out in accordance with applicable regulatory requirements.
In the auditor's view, there is nothing to prevent further cooperation.
22.08.2013 Lichtenheldt
The audit at GUS ERP GmbH, Cologne site, was successful.
Overall verdict: GOOD
From the auditors' point of view, nothing stands in the way of further cooperation.
21.03.2013 Stoma
[...]The results demonstrate the necessary experience and GMP competence of the company for the development, implementation and maintenance of the OS-ERP software. The company was able to demonstrate in the audit that customers from the GxP-regulated industry have carried out successful audits in recent years. The company was able to demonstrate that its extensive experience in the pharmaceutical industry underpins its expertise. [...]
The employees have many years of experience in the GxP market, a high level of understanding of the validation requirements and identification with the company. Compared to the ERP market (DACH), GUS is very well positioned to meet the requirements of the GxP regulated industry.
31.07.2012 BGS
Conclusion: The very good impression gained during the offer and project phase was confirmed in the audit. Strength: GUS maintains and lives an appropriate and well-documented quality management system in which the costs and benefits appear to be in a very good relationship. No deviations were found. GUS should be approved as a supplier for the quality-relevant product ERP system GUS-OS.
01.12.2011 Steigerwald
The implementation of assured processes - initiated by the certification - particularly in the areas of product development and project implementation shows that the company pursues an active quality policy and does not just react to customer requirements. [...]
The auditors gained the impression that the quality assurance system presented is practiced and implemented in all relevant areas of the company. [...]
With regard to the use of standard solutions in the pharmaceutical GxP sector and the associated services, GUS is classified as a qualified supplier.
23.11.2011 bela-pharm GmbH & Co. KG
The audit was conducted in an open and friendly atmosphere. The requested information was available at short notice and the staff was available to answer questions competently. The GUS QM system made a well thought-out and transparent impression. The special requirements of the pharmaceutical environment were obviously known and were fulfilled accordingly.
07.06.2011 Tillots Pharma AG
The audit team received confirmation that GUS has anextensive and well-applied Quality System in place. Development methods and testing standards are defined and applied. Product release follows predefined methods. Change control and configuration management are handled skillfully. Document review and approval is clearly established for QMS documentation.
19.06.2009 AvidiaMed
The strengths of GUS certainly lie in its consistent focus on quality over many years. Quality awareness and the associated QM system have been continuously developed and internalized. The fact that the benefits of quality have been recognized and implemented was communicated by employees and management throughout the entire audit. The focus on quality was demonstrated by the current QM system and the tool-supported documentation of system and development tests (e.g. VALREP). The audit confirmed that GUS develops software and provides services in accordance with GxP requirements.
17.02.2009 SANIPharma GmbH
The quality management system of GUS ERP GmbH is exemplary and of a high standard. This is demonstrated, among other things, by the regular certifications in accordance with DIN EN ISO 9001, as well as the transparency and sovereignty on the part of GUS during the audit. No corrective measures required.
18.08. - 21.08.2008 medac Gesellschaft für klinische Spezialpräparate GmbH
Results Overall assessment:
GUS Development Lifecycle processes and procedures are VERY GOOD
GUS staff are all competent, good listeners, open to change, know their business
GUS team appears to be committed to the overall success of the joint Medac/GUS Project
auditors feel that GUS has the ability to compete in the US market (and worldwide)
Pluses
- Training Documentation availability on the intranet
- Configurability of the system
- Audit trail functionality
- Cross functionality
- Visualization of BOM level to review status, deviations, etc.
- Quality Systems
- Ability to generate the documentation of roles, workflows, etc needed for IQ
- Real-time database for capturing errors and problems
Assessment documents were generated in the following areas:
Review of Quality Systems, Development and Production Life Cycle Supporting GUS-OS ERP
21 CFR Part 11 Assessment (Electronic Records/Signature) for GUS OS ERP
GMP suitability of GUS OS ERP software functional requirements
20.11.2007 Parexel
The GUS group is trusted providing software with high quality. The company is also trustedto deliver appropriate services to its customers, and to continue to do so in future. This isproven by the company's history, the large customer base, the high number of installations,and the loyalty of customers.
The staff is trusted to be competent, knowledgeable, and well trained as proven by (most) personal files.The Quality Management system including the QM handbook and all documentedprocedures were impressively presented. The ISO certification provides evidence that this is agreed by certifiers.
13.06.2007 Hennig Arzneimittel GmbH
"During the audit, the company management, all directly and indirectly involved employees from development, project and service conveyed absolute knowledge and application of the QM system and that this is also really lived.
The following results from this audit are to be noted:
- Strength of the company:
The strengths of GUS certainly lie in the consistent focus on quality for many years. Quality awareness and the associated QM system have grown and been internalized. The benefits of quality have been recognized and implemented. This has been communicated throughout the audit to the current QM system, the tools used by the company (e.g. VALREP) as well as to the employees and the management. The audit has confirmed that GUS develops GMP compliant software and provides services. - Potential for improvement/recommendation:
No significant weaknesses were identified in the course of the audit.
03.08.2006 Bombastus-Werke AG
"GUS ERP GmbH was able to prove that the GUS-OS ERP system was developed using a mature quality management system.
The quality management system covers the GMP requirements for the development of software.
A significant factor for the quality is the distinctly high level of qualification and experience of the employees, the untypically long industry experience and the references in the pharmaceutical industry."
03.03.2006 Interested party
"The quality management system presented in the course of this audit is suitable for ensuring traceable software development, for documenting errors and their elimination in a traceable manner and for processing them in a controlled manner, and for enabling the proper, largely prepared introduction of the GUS-OS ERP software at the customer."
"With GUS-OS ERP, it should be emphasized in particular that the customer's validation activities are supported by comprehensive test documentation already created during development, as well as a validated documentation tool VAL-REP for recording test cases."
"No critical, serious or other deficiencies were identified in the audit."
07.12.2005 Riemser Arzneimittel AG
"The GUS has installed a QM system that is suitable for ensuring the creation of software for the pharmaceutical sector that meets the requirements of GMP."
"Particular emphasis should be placed on the great efforts that the GUS Group has made to ensure that the GUS-OS ERP meets the requirements of the pharmaceutical industry for documentation and validation of such a program. Likewise, the wide range of assistance that can now be offered to these companies for validation."
"The GUS Group can be approved as a software supplier for the pharmaceutical sector."
Annual appraisal for system promotion
Once a year, DQS reviews our quality management system in an "assessment for system promotion". The quality management system, document control (not public), the implementation of specifications in practice and employees' knowledge of process descriptions (not public), specifications and guidelines are reviewed. We have also received and successfully implemented numerous suggestions for process and quality optimization.
> DQS certificate for download
Expert opinion
For the verification of development work and process management tasks, we commission external experts at irregular intervals to prepare expert opinions. Especially with the new introduction of the GUS-OS solution family, the development processes and customer projects were subjected to an extensive external review.
(Version 1.3 from 16.01.2018)
Evaluation of the management system
The basis for the evaluation of our company's key figures and information are our company's targets or the comparison with figures from previous reporting and evaluation periods as well as planning data. In the event of an accumulation of errors, a tendency for results to deteriorate, or as part of our continuous improvement activities, measures are initiated to eliminate the causes of errors or deterioration. Evaluation of the management system takes place at several levels: Evaluation by the management circle (management reviews)In regular management reviews, commercial and quality-related data of the company are analyzed and discussed, which allow conclusions to be drawn about the performance of the company's processes. Inputs for the management review are:
- Results of internal audits,
- Results of supplier audits,
- Feedback from customers (reports to the hotline),
- Process metrics,
- Economic target figures,
- Target/actual comparisons with plan data,
- Status of corrective and preventive actions,
- Changes that affect the management system,
- Recommendations for improvements.
- Feedback from relevant interested parties
- Results of monitoring and measurements
- Services from external providers
In the event of anomalies, suitable measures are initiated in order to respond as promptly as possible to the weaknesses identified in the company. The results and determinations of the assessment are recorded and communicated to those responsible.The implementation of agreed measures is monitored by the quality officer and the management circle.
Evaluation by the top management
We conduct a formal evaluation of our management system once a year. The basis for this is the report of the Quality Management Representative. Inputs for his report are:
- Results of internal audits (not public),
- Results of supplier audits,
- Feedback from customers and reports to the hotline,
- Process metrics (non-public).
Furthermore, the report contains information on
- Status of corrective and preventive actions,
- Changes that affect the management system,
- Recommendations for improvements.
The results and determinations of the assessment are recorded and communicated to the responsible parties. The implementation of agreed measures is monitored by the quality management representative and the management circle.
Corrective and preventive measures
The control and monitoring of corrective and preventive actions (not public) is carried out by the QM organizational unit.
Corrective action
Deficiencies in the organization are identified at an early stage through regular observation and evaluation of the data obtained in audits. Those responsible for the departments have a duty to identify systematic deficiencies within the scope of their responsibility and competence and to remedy them immediately or report them to their superiors. Accumulations of errors are identified, and the causes are eliminated through appropriate measures. Small corrective measures are implemented directly and unbureaucratically by those responsible. Extensive corrective measures, in which, for example.
the repeated occurrence of critical errors is to be counteracted,
cross-departmental or cross-company measures are necessary,
far-reaching investment or organizational decisions are to be expected,
are coordinated with the management level. Depending on the scope of the measures, they are systematically planned, documented and implemented. Corrective measures that affect existing regulations of our company are entered accordingly in the documentation of our management system. The effectiveness of corrective measures is reviewed.
Preventive measures
Preventive measures are introduced to minimize or eliminate expected risks in products or processes with regard to quality-relevant effects before problems arise. They are based on experience gained from known, comparable processes and procedures and are incorporated into the planning and development of new products and the improvement of existing processes or procedures. The effectiveness of preventive measures is reviewed.
(Version 1.2 from 25.10.2013)
The quality management representative is equipped with the authority to decide on all quality-relevant actions regarding our quality management system. He has the task of maintaining and further developing our QM system, which is certified according to the DIN EN ISO9001 standard. Furthermore, he is responsible for the control of an efficient implementation of the quality-relevant instructions. In addition, he is responsible for the training of employees in order to sensitize them to the issues of quality management. The Quality Representative acts in his area of responsibility free of instructions and independent of superiors. Tasks:
- Establishment, maintenance and further development of the management system according to DIN EN ISO9001
- Review of QM pre-commissioning documents (preparation by the process owners)
- Updating and monitoring of corrective and preventive actions (development by process owner)
- Implementation of supplier audits
- Implementation of internal audits
- Training of employees on the QM system
- Appointment certificate QM representative (not public)
(Version 1.7 vom 04.06.2024)
GUS QM Manual
Die Unternehmensleitung der GUS ERP GmbH setzt dieses Handbuch mit dem heutigen Tag in Kraft und legt fest, dass die darin enthaltenen Anweisungen sowie zugehörige Dokumente für die Mitarbeiter des Unternehmens verbindlich sind.
Köln, den 04.06.2024
Thorsten Schlechtriem, CEO of GUS ERP GmbH